 What Is HELLP Syndrome? How Can It Affect Mothers and Babies?
What Is HELLP Syndrome? How Can It Affect Mothers and Babies?Warning: The NCBI website requires JavaScript to operate. Preeclampsia: Long-term Consequences for Vascular Health Lorena M AmaralDepartment of Pharmacology, University of Mississippi Medical Center, Jackson, MS, USAMark W Cunningham, JrDepartment of Pharmacology, University of Mississippi Medical Center, Jackson, MS, USADenise C CorneliusDepartment of Pharmacology, University of Mississippi Medical Center, Jackson, MS, USABabbette University of Mississippi Pharmacology This disease is characterized by the new hypertension of beginnings generally in the third trimester of pregnancy and is sometimes associated with proteinuria, although proteinuria is not a requirement for diagnosis of PE. In developing countries, women have a higher risk of death due to EP than the most affected countries and one of the most common causes of death is high blood pressure and stroke. Although the PE only affects about 2%–8% of pregnancies around the world is associated with severe complications such as eclampsia, hemorrhagic stroke, hemolisi, elevated liver enzymes and low platelets (HELLP syndrome), kidney failure and pulmonary edema. Importantly, there is no "cure" for the disease except for early delivery of the baby and placenta, leaving PE a risk of medical care for babies born of PE mothers. In addition, PE is linked to the development of cardiovascular diseases and strokes in women after reproductive age, leaving PE a risk factor for long-term health in women. This review will highlight the factors involved in PE physiopathology that can contribute to long-term effects on women with pre-eclampthic pregnancies. Introduction The underlying pathophysiology of preeclampsia (PE) is not fully understood, but it is currently believed that the starter event in PE is reduced the placental perfusion, which develops from the shallow migration of cytophoblast to the uterine spiral arteries leading to inappropriate vascular remodeling and a hypopertinous placenta. This placenta becomes ischemic as the pregnancy continues, leading to the release of factors that cause endothelial maternal dysfunction. Endothelial dysfunction results in widespread vasoconstriction, blood reduction to multiple organs and has been a major phenotype of PE. – In addition, pre-existing conditions such as poor nutrition, diabetes and obesity are risk factors for PE, and could exacerbate the maternal response to factors released from ischemic placenta. While 800 women die from pregnancy complications around the world every day and 3 million premature births reported every year are related to EP, there is no effective treatment for this pregnancy disease, except for early delivery of the fetus. The cardiovascular and maternal kidney system passes through several important adaptations during a normal pregnancy. Heart output, heart rate, and stroke volume increases during pregnancy due to increased plasma volume expansion and systemic vasodilation during pregnancy. The lack of these changes during pregnancy is usually associated with pregnancy complications and a higher risk of developing cardiovascular events (such as myocardial infarctions, venous thromboembolisms and strokes) in the mother later in life. Multiple clinical studies of women with PE show a higher risk of developing cardiovascular diseases later in life. For example, a Norwegian study from 1967 to 1992, with more than 600,000 pregnant women, showed that women with PE have an 8-fold increase in death as a result of a cardiovascular event against women who have a normal pregnancy. A study of 30,000 women in the state of Washington from 1967 to 1998 showed that women with mild and severe PE had a double and 3 times higher risk of cardiovascular events later in life, respectively. In addition, a 30-year follow-up study of √14,000 women in California noted that women with PE have a double death increase due to cardiovascular events, and women who had the start of PE before 34 weeks of gestation had a 9-fold increase in life. Finally, a Taiwanese study of 1 million women shows that women with PE have a 12-fold increase in having a cardiovascular event. Thus, all of these clinical studies performed together show that women with PE have a higher risk of having a cardiovascular event later in life regardless of the place, indicating the importance of the common mediators of this disease shared among these women to help identify the link between PE and subsequent cardiovascular events. Renal insufficiency is a common thread that could contribute to long-term pathology of previous preeclampthic women.Despite an increase in heart production during pregnancy, there is a decrease in maternal blood pressure due to a decrease in total peripheral resistance caused by maternal vasodilation during pregnancy. In addition, in the kidneys, the glomerular filtration rate (GFR) and renal blood flow increase due to a decrease in renal vascular resistance. Women with PE have often altered kidney function, glomerular endotheliosis and proteinuria. The first studies conducted in the 1970s by Gibson showed that the lack of increase in GFR during early pregnancy is associated with women who have a higher risk of unexplained births, abortions or small for gestational-age babies. A population study composed of Norwegian pregnant patients from 1967 to 1991, showed that 477 patients of 570,433 pregnant women developed final-stage kidney disease (RDS). Of the 477 women who developed ESRD, the authors found that women who had been pregnant once or more and developed PE during the first pregnancy are four times more likely to develop ESRD. They also showed that as the number of recurring preeclampthic pregnancies in the mother increased, the higher the risk of developing ESRD. Importantly, there is a decrease in GFR and proteinuria in preeclamptic patients. Several studies show that podocitoturia, which is the urinary excretion of podocytes, rises in women with PE. The amount of podocytes, podocytes in the urine, can be determined by quantifying the protein structures podocalyxin, podocin, nephrine and sinaptopodine in the urine.– A more recent study by Garovic et al showed that podocyturia had a higher predictive value for the diagnosis of PE against other angiogenic factors, such as soluble fms such as kinase-1 thyrosine (sFLT-1), endoglin and placentero growth factor (PlGF). It is important to note that the loss of podocytes, which have a very limited regenerative capacity, from glomerulus can lead to increased proteinuria and glomerulosclerosis due to the interruption of the glomerular filtration barrier. Therefore, podocituria can serve as a sensitive biomarker for the development of PE and the degree of kidney damage observed in PE. Endothelial dysfunction is a common thread that could contribute to the long-term pathology of preeclamptic women previously nitric oxide (NO) suprathelial dysfunction is characterized by an increase in ET-1, a potent vasoconstrictor, secretion of endothelial cells and lack of vascular responses appropriate to mid vasodilators. While the mechanisms responsible for maternal systemic vascular dysfunction are unknown, it has been shown that mediators of endothelial dysfunction as the decrease of NO play a role in the development of hypertension in preeclampthic women. Previous studies have shown that sinthesis inhibition NO in pregnant rats is associated with intrauterine growth delay (IUGR) and L-arginine supplementation prevents the restriction of fetal growth in animal models of IUGR. In addition, supplementation with L-arginine in pregnant women of IUGR improves vasorelaxation by indicating that this pathway could be an important role in IUGR physiopathology in human pregnancies. NO, originally identified as the endothelium-derived relaxation factor, is an important synthesized vasodilator in response to mechanical and chemical stimuli of L-arginine by a family of calcium-calmodulin-dependent enzymes, called nitric oxide syntase (NOS). DO NOT induce vascular smooth muscle relaxation through guanylate soluble ciclase (sGC)/ guanosine cyclic monophosphate (cGMP)-dependent and independent mechanisms. In addition, it does not inhibit the adherence of leucocytes and has antitrombotic and antiapoptotic effects. NOT derived from the endothelial nitric oxide synthesis (eNOS) is an important mediator of vascular homeostasis. It has been shown that the subregulation of eNOS, which has led to an increase in NO production, contributes to increased uteroplacental blood flow through changes in the vascular tone. In addition, there is evidence that eNOS genetic polymorphisms can affect susceptibility to hypertensive disorders of pregnancy., Alterations during normal pregnancy, such as increased blood volume is hosted within the cardiovascular system by systemic vasodilation associated with NO production, suggesting that the deficiency could NOT be an important role in hypertensive disorders of pregnancy. In fact, several studies in human and animal models have shown that the vascular relaxation deteriorated in PE has been attributed to the reduction of the bioavailability of NO produced through eNOS., previous studies have shown a high expression of ENOS during PE, however both increases of the arginasse expression (NOS regulation) and high levels of a natural inhibitor of NOS, the asymptoarginine vasoglow After 24 hours, after delivery, these levels did not change in the normal pregnant group, however, nitrate-nitrito levels (NOx) increased in the preeclamptico group supporting the idea that alterations in regulation can NOT play a key role in the PE. Experimental animal models of placental ischemia have been shown, including reduction of uterin perfusion pressure (RUPP) to exhibit impaired vasorelaxation in tubes and the activity of NO has been investigated., In addition, previous researchers have demonstrated a loss or absence of vasorelaxation mediated by NO in glasses treated with RUPP plasma. In our recent study we have shown that circulating NOx levels and eNOS vascular expression were decreased in RUPP rats. Although it is necessary to investigate more thoroughly the benefits of NO, drugs that point to NO components can increase bioavailability DO NOT contribute to improving maternal outcomes such as hypertension and endothelial dysfunction. In addition, endothelial dysfunction markers such as ET-1, soluble vascular adhesion molecule and IL-8, or endothelial leukocyte adhesion molecule 1 (ELAM-1), which could be elevated weeks before the clinical manifestation of PE. In fact, detection of endothelial dysfunction or vasoconstriction can serve as a predictor of this disease and can be useful to understand the pathways associated with PE. Peripheral arterial tone and other methods that depend on mediated flow dilation have been used to evaluate endothelial function during pregnancy and PE. Although peripheral arterial tone did not identify women who will develop PE, this technique could be associated with a relative peripheral vasoconstriction in pre-eclampthic women after delivery and could be used to detect anomalies that persist after pregnancy. In addition, peripheral pulse pressure waves determined by application tonometry method demonstrate changes in the form of radial artery pulse wave and its correlation with central aortic pressure and pulse wave during pregnancy. In fact, tonometry has shown a high rate of increase in preeclampthic women compared to normal pregnant women. Non-invasive vasoconstriction measurements during pregnancy in future studies can help predict PE and the risks of post-delivery cardiovascular disease.s Flt-1The abnormalities in the placenta are associated with increasing anti-angigen factors such as the receptor of the vascular endothelial growth factor sFlt-1 or soluble that contribute to reducing kidney function and hypertension during pregnancy. Preeclampthic women have increased the circulating Flt-1 and placental sFlt-1 mRNA levels compared to women who have normal pregnancies. In addition, the effects of pro-angigen VEGF and PlGF factors that are important to maintain vascular endothelium are antagonized by sFlt-1 which is a possible cause of endothelial dysfunction during this disease. Previous studies have shown that VEGF and PIGF levels decreased in correlation with higher sFlt-1 levels during PE. In addition, VEGF is important to induce and maintain the integrity of the fenestrated endothelium in various tissues, including renal glomerulo, and the VEGF block by sFlt-1 may be a cause of kidney damage and a decrease in kidney function. The role of SFlt-1 in the pathogenesis of this hypertensive disorder during pregnancy has been supported by animal data. When given to pregnant and non-pregnant rats using an adenoviral vector, sFlt-1 induces PE syndrome that results in classic signs such as hypertension, proteinuria and glomerular endotheliosis. Recent studies by Murphy et al show that sFlt-1 infusion in normal pregnant rats caused hypertension that was associated with endothelial dysfunction characterized by an increase in ET-1 and NO decrease. Importantly, L-arginine improved hypertension and ET-1 expression. These data further support the hypothesis that the increased availability of NO can be an innovative way to better treat PE treatment. Importantly, a recent document has shown that the sFlt-1 levels were normalized after 2 months after delivery in exposed PE compared to control mice. In the same study, an improved vascular response to lesions in vessels exposed to PE was observed after delivery. In addition, women who develop PE did not show differences in serum sFlt-1 levels to 12 weeks in postpartum compared to their third quarter. Therefore, high sFlt-1 levels during and after PE could play a persistent role in endothelial dysfunction and damage after PE. In addition, an altered expression of angiogenesis-proteins was presented in women who had before PE more than 1 year after delivery. Although PE is resolved by delivering placenta, and altered angiogenesis and vascular damage have been associated with long-term cardiovascular risk, it is necessary to undertake more extensive studies to verify whether postpartum measures of sFlt-1 levels have a role in predicting future cardiovascular diseases. Chronic inflammation is a common thread that could contribute to the long-term pathology of previous preeclampthic womenDuring normal pregnancy, the fetus must overcome immune rejection by the maternal immune system. Therefore, tolerance should be established for fetal semitologenic antigens, especially in the maternal-fetal interface, while maintaining immune protection against pathogens. In the maternal-fetal interface, leucocytes constitute 30%-40% of the decidual cells and are composed mainly of NK cells, CD14+ myelomonocytic cells and T lymphocytes. Normal pregnancy occurs with mild inflammation, however, women with PE have chronic immune activation and have an exaggerated innate inflammatory response. Multiple studies involve a number of immune factors in mediating hypertension and endothelial dysfunction during pregnancy. Clinical studies have shown an increase in the production of pro-inflammatory cytokines in PE compared to normal pregnancy, and these data are also observed in animal models of PE., – T lymphocytes are instrumental for immune memory and can play a role in the long-term sequel of PE, such as stroke or other cardiovascular events. CD4+ TCD4+ support cells T cells are a heterogeneous group of cells composed of several different subsets to include TH1, TH2, TH17 and CD4+ TReg cells. Clinical studies have shown that populations of all CD4+ subsets T cells do not increase, but rather TH1 and TH17 subsets increase while TH2 and TReg subsets are decreased in women with PE compared to women with normal pregnancies.– These changes in CD4+ cell populations are described as a CD4+ The imbalance of T cells and recent studies have partially determined how changes in these specific populations contribute to physiopathology during PE. TH1 cells produce IL-2 and IFN-γ, are pro-inflammatory, and are involved in cell immunity, while TH2 cells produce IL-4, IL-5, and IL-13, are anti-inflammatory, and are involved in humoral immunity. Normal pregnancy is associated with a predominant TH2 profile and suppressed TH1-type immunity, while a higher proportion of TH1:TH2 cells is observed in PE., Zenclussen et al. made adoptive transfer of active BALB/c TH1-like specimens in female BALB/c mice in late gestation. This resulted in the development of a model similar to PE that has higher blood pressure, glomerulonefritis and proteinuria. In addition, in response to the adopted transfer of activated TH1-type cells, the production of cytokines and fetal reabsorptions was also increased. However, the adoptive transfer of these cells to unpregnant mice did not produce any pathological response. This study shows that the increased activation of the TH1 cell population causes physiopathological symptoms only during pregnancy. In our studies with the PE RUPP rat model, the T-cell profile is similar to that seen in PE patients, RUPP rats have an increase in CD4+ T-help cells characterized by the increase of TH17 and the decrease of TRegs. We showed that the adoption transfer of RUPP CD4+ T cells in normal pregnant rats increase blood pressure and reduce kidney function. In addition, RUPP CD4+ T cells cause placentero and renal oxidative stress and increase ET-1.,, We believe that a T-cell-stimulated mechanism is to facilitate the production of AT1-AA B cells, as discussed in greater detail below and is seen in .The plasma ischemia is a stimulus for chronic inflammation that leads to vasoactive factors that could play a role in later CVD in preeclampthic women. Abbreviations: RBF, renal blood flow; GFR, glomerular filtration rate; CVD, cardiovascular disease; ROS, reactive oxygen species. Several clinical studies have reported that the population of TH17 cells is significantly increased in the PE compared to normal pregnancy., Recent preclinical studies have begun to elucidate a role for TH17s and IL-17 to cause much of the physiopathology observed in PE., TH17 cells are distinguished from other populations of CD4+ Cells T by their intracellular secretion, superficial expression of the recellular acid. Studies have shown that increased inflammation observed during the PE persists. Vitoratos et al proved that women with PE remained under inflammatory stress up to 12–14 weeks after delivery. A later study of Kvehaugen et al showed evidence of chronic systemic inflammation and persistent endothelial dysfunction 5-8 years after delivery in women and their offspring after PE. These data suggest that persistent, postpartum inflammation may play a role in the future risk of cardiovascular disease (CVD) in women with PE. In addition, a preclinical study of Pruthi et al showed that exposure to experimental PE led to an increase in vascular damage after injury compared to normal pregnancy in mice. Vascular dysfunction observed in preeclamptic postpartum women may be due to persistent inflammation after delivery. However, further studies are still needed to determine this in a definitive manner. In addition, studies to determine if the inflammatory assistant CD4+ T-cell populations continue to be elevated after delivery in preeclameptic women. Several clinical studies show that, in contrast to the increase in the population of inflammatory cells, such as TH17, a decrease in the number of CD4+ TRegs occurs in preeclampthic women compared to their normal pregnant counterparts. ,– CD4+ TRegs are classically identified by the superficial expression of CD4 and CD25, as well as the intracellular expression of the transcription factor of the P3 (FoxP3). The TRegs are responsible for maintaining maternal immune tolerance during pregnancy. Maternal immune tolerance depends on NK TRegs and uterine cells recognizing and accepting fetal antigens and facilitating placental growth. Deintegration in maternal and fetal tolerance is considered to be a mediation factor in the development and pathology of persons with disabilities. Previous studies have shown that the depletion of TRegs before conception or early pregnancy resulted in failures of implantation and increase of resorptions. Therefore, the importance of TRegs in the maintenance of early pregnancy has been previously established. However, the maintenance of the TReg of the proper immune function can also be important in mid-to-after pregnancy. Due to the roles of the over-stimulated immune response in the cause of PE physiopathology, the immune regulation appropriate for TRegs can be important to unravel inflammatory mediators that lead to vasoactive pathways that can cause hypertension and PE symptoms. CD4+ TRegs may inhibit endogenous activation of TH1 and TH17 pro-inflammatory cells that lead to a decrease in inflammation and oxidative stress. Without the activation of T cells, inflammatory cytokines production would be inhibited, resulting in fewer inflammatory cells and less production of reactive oxygen species. To date, no study has investigated the possibility of a long-term autoimmune response after PE involved CD4+ T-cells or B-cells. In addition, very few studies have investigated the risk of PE that leads to other autoimmune diseases later in life. To date, two such studies have identified a slightly higher risk of rheumatoid arthritis (RA) in women previously diagnosed with PE. A national cohort study in Denmark examined the potential effects of live births, loss of pregnancy and complications of pregnancy on the risk of development of the RA later in life. These studies in Danish women found that the rate of RA was 1.42 in women previously diagnosed with PE compared to women with normal pregnancies, while a recent update reported the risk ratio of RA in women diagnosed with PE to 1.96 compared to 1.18 in women diagnosed with gestational hypertension, without PE, Although these risk coefficients are relatively small, the deregulation of the immunitarian system of preeclamptica women can lead to the subsequent disease. Studies continue to be needed to investigate an association between PE and a higher risk for other auto-inmunitive disorders, especially those that disproportionately affect women and are associated with cardiovascular diseases such as systemic lupus erythematosus and psoriasis. NKNK cells make up 10% of lymphocytes in human peripheral blood and are identified by their CD56 expression in the absence of CD3. NK cells can be differentiated in two different subsets, type 1 (NK1) or type 2 (NK2). The NK1 subset is characterized by its release of IFN-γ and powerful cytolytic activity when activated., IL-2 and IL-12 signaling promote the differentiation of NK cells to the NK1 subset and are secreted by CD4+ Help cells. CD4+ TRegs may inhibit the mediated differentiation of IL-2 by decreasing the availability of cytokine., It has also been shown that IL-17 can improve cytolytic activity of NK cells, suggesting that TH17 cells can play a role in mediating differentiation in the NK1 population subset. PE is associated with a change in NK2 cellular population to NK1 (type 1). A prominence of NK1 cells that secrete the alpha tumor necrosis factor (TNF-α) has previously been shown in pre-eclampthic women. NK-mediated death of fetal trophoblasts can contribute to the shallow invasion of trophoblasto resulting in the insufficient remodeling of the spiral artery. Promoting the development of the ischemic placenta, which leads to the release of vasoactive factors that mediate maternal vascular dysfunction. Recent studies in mice suggest a role for cytotoxic NK cells in Angiotensin II (ANGII) mediated vascular dysfunction and artherosclerosis.– In addition, a human study demonstrated an association with NK cells and a breakup of growing plates., The role of the cytolytic cell population NK1 in the development and physiopathology of PE and other cardiovascular diseases is misunderstood and warrants further research. Neutrophils Neutrophils are activated in response to hypoxia and placental inflammation occurring during the PE. These cells can also be recruited and activated by the increase of IL-17. These cells can contribute to the increase of vascular resistance and the fetal morbidity of PE through the production of oxidative stress and the release of extracellular neutrophil traps (NET). Networks are extracellular structures released from neutrophils that kill extracellular bacteria and fungi, but are involved in pathogenesis of inflammatory disorders and autoimmunity., Inflammation caused by oxidative stress and damage and vascular dysfunction can be attributed in part to the activation of neutrophils and the subsequent release of the networks in response to hypoxia and PE-17. ,AT1-AAAT1-AAs are elevated in women with PE., AT1-AAs unite with a high affinity to the sequence of 7 amino acids in the second extracellular loop of AT1 The binding of AT1-AA to AT1R, increases the receptor activity AT1, the intracellular levels of calcium and activation of the kinase/inase cellular protein The AT1-AA also rise in normotensive pregnancies with uterine-restricted fetuses, kidney transplant receptors and patients with systemic sclerosis, vasculopathy, tissue fibrosis, hypertension, renovascular disease, and pregnant women with hemolisis, elevated liver enzymes and low platelets (HELLP syndrome).– In 1999, Wallukat et al was the first to identify AT1-AAs in women with PE and not in healthy pregnant women or in women with pre-existing hypertension and essential pregnancies. In this study, the AT1-AA were isolated by affinity column purification techniques and used to stimulate cultivated neonatal rat cardiomyocytes. However, in the presence of losartan, an AT1R blocker, purified AT1-AA of preeclampthic patients could not stimulate cultivated cardiomyocytes. Therefore, this study was the first to identify that AT1-AA actions on cardiomyocytes are facilitated by AT1-AA binding on AT1R. In vitro experiments with the AT1-AA on vascular smooth muscle cells and trophoblast cells increased the production of reactive oxygen species, nicotinemide dinucleotide components of oxidase phosphate and activation of activated kappa-light-chain-enhancer nuclear factor. The smooth muscle cells of the human coronary artery incubated with AT1-AAs increase the levels of tissue factor, which rise in the placenta of preeclampthic patients. AT1-AAs administered to human throphoblast cells, increased the secretion of the plasminogen-1 activator inhibitor and decreased trophoblast invasivity. Together these effects on trophoblast cells suggest a placental placentation mechanism, a possible cause of PE. Human mesangial cells incubated with plasminogen-1 activation inhibitor and IL-6 secretions. AT1-AAs cultivated with cardiomyocytes, increase the rate of beating of cardiomyocytes and cardiomyocyte apoptosis through a TNF-α pathway. In addition, we have shown that endothelial cells of the human umbilical vein (HUVECs) incubated 6 hours with ANGII (10−7 M) and AT1-AA expression increased drastically. This 100-fold increase in the ET-1 expression was absent in HUVECs incubated with ANGII or AT1-AAs alone, suggesting a possible improvement in ANGII signaling when AT1-AAs are present. Therefore, the increase in HUVEC's ET-1 expression can play a significant role in enhanced hypertension and ANGII sensitivity during PE. The results of the HUVEC study further emphasize the importance of the ET-1 expression in PE, where the production and hypertension AT1-AA is increased. In a previous study we showed that the blocking of ETa receptors in rats administered by AT1-AA and ANGII showed a decrease in blood pressure. Thus, this in vitro HUVEC data taken along with in vivo data strongly support the notion that ET-1 is an important hypertension mediator during pregnancy. PE animal models, such as the RUPP model, transgenic human angiotensinogen and the model of a pregnant rat of renin genes, the adoption transfer of CD4+ TNF-α, IL-6 and IL-17 lymphocytes manage during the pregnancy model, show elevations in AT1-AAs during pregnancy., – AT1-AAstages in mice-admitted stress increases. EMPs are small fragments of endothelial cell debris that rise in women with PE and serve as a marker of endothelial cell dysfunction. The mechanism by which AT1-AA increases the EMPs is through the transduction route of signaling the p38 MAP kinase. In addition, the AT1-AA administered to pregnant rats on the 12th or 13th day of pregnancy showed a remarkable increase in blood pressure on the 19th day of pregnancy., Rats also show and increase placental oxidative stress, and the ET-1 expression in the renal cortex, aorta and placenta.,The most sensitive ANGII mechanism in PE is unknown. However, we hypothesize that AT1-AA play an important role in increasing ANGII sensitivity in PE. Studies of our laboratory have shown that acute ANGII along with AT1-AA infusion during a rat pregnancy increased blood pressure. In addition, chronic ANGII and AT-AA increase blood pressure, oxidative stress, and ET-1 secretion and increase the renal artery resistant index above ANGII or AT1-AA administered separately., A mechanism by which AT1-AA can increase ANGII sensitivity can be by altering ANGII binding affinity to AT1R. Preliminary studies of our laboratory have shown that AT1-AAs increase (abundant 15 times) the binding affinity of ANGII to AT1Rs in HUVECs incubated with fluorescent ANGII and human AT1-AA insulated preeclamptics patients for 1 hour. This increase in the ANGII union was also correlated with an increase in ET-1 secretion. Another mechanism proposed by which AT1-AAs increase ANGII affinity to AT1R is by increasing the decimization of AT1 receptors. Multiple studies have shown an increase in the response capacity of AT1R to ANGII, when the AT1R heterodimerizes with the vasodepressant bradykinin receptor (B2).– AbdAlla et al showed that women with PE have increased amounts of B2 protein and AT1/B2 heterodimerization in platelets and omental vessels of preeclampthic patients.– Therefore, it can be postulated, that the process by which AT1-AAs increase the sensitivity of ANGII in PE is mediated by the AT1R receptor dimerization, however, further studies are needed to verify this hypothesis. Studies conducted by Hubel et al show that 18% of preeclampthic women postpartum have increased the circulation of AT1-AAs 1 year after delivery. These women with high AT1-AA have high levels of sFlt-1, decreased VEGF and increased insulin sensitivity. The correlation between the increase of AT1-AA and these variables can suggest a mechanism by which women with PE have a higher risk of cardiovascular events later in life. With caution, this study consisted only of 64 women (35 controls and 29 women preeclamp-tic), in which ~18% (five of 29) of pre-eclampthic women postpartum had increased the circulation of AT1-AAs, indicating the importance of studies that determine the importance of AT1-AA with higher populations of pre-eclampthic women postpartum. Rats administered AT1-AA during pregnancy showed no differences in heart function compared to normal 16 weeks postpartum pregnant rats in a study conducted by Wang et al. However, in this study there were several morphological changes in cardiomyocytes, increased collagen content and changes in the myocardial structure of rats administered by AT1-AA during pregnancy. These changes were prevented when the slatman was administered along with AT1-AA during pregnancy. These data together suggest that AT1-AA can play a key role in the development or pathogenesis of cardiovascular dysfunction and CVD in the mother. PE has long-term effects on offspringNot only PE has a long-term effect on the mother's cardiovascular system later in life, but it also has an effect on the cardiovascular system of offspring later in life. PE is one of the main contributors to premature and low-weight babies at birth. For several years, especially with the development of Barker's hypothesis, this fetal adaptation during pregnancy, such as malnutrition caused by hypertension and/or placental ischemia during pregnancy, increases the risk of the child's development of hypertension, stroke, diabetes and CVDs later in life. In addition, several recent studies of metaanalysis have shown that for every 1 kg increase in cardiovascular mortality, there is a decrease of 15% The offspring of women with PE has high blood pressure, body mass index, and increased triglycerides and cholesterol content in both adolescence and adulthood., A meta-analysis study conducted by Davis et al in 2011, examined more than 18 cohorts of women with normal and hypertensive pregnancies. In this study, they discovered an increase of 2.39 mmHg in systolic pressure, 1.35 mmHg in diastolic pressure, and an increase of 0.32 kg/m2 in the body mass index in the descent (4-30 years) of preeclampthic mothers against the offspring of normal pregnant women. Conclusion The clinical symptoms of PE can be resolved after the birth of the placenta, however women and their descendants affected by PE have a double risk of subsequent cardiovascular complications, such as heart disease, strokes and venous thromboembolism during the 5-15 years after delivery, and these women have greater risks of dying from brain disease after pregnancy than women who had a healthy pregnancy. A more recent study examined the incidence of long-term atherosclerotic morbidity in preeclampthic women and the risk is greater for patients with severe and recurrent episodes of PE. In addition, endothelial dysfunction is a common feature of pregnancies with PE, atherosclerosis and cardiovascular diseases, and therefore endothelial dysfunction could serve as an underlying mechanism for the development of cardiovascular diseases in preeclampthic women or their offspring. In addition, PE women have chronic indicative immune memory inflammation with the activation of CD4+ aid cells T and the secretion of IgG specialized in the form of AT1-AA. The prevalence of AT1-AA, immunologic memory with NK or T cells and the sequel to endothelial dysfunction could mediate the development of CVD or cardiovascular events later in life among PE women. In addition, due to this prolonged chronic inflammation, PE women may be at risk for autoimmune diseases later in life. In recent years, several studies or small cases have been published suggesting the use of monoclonal antibody, rituximab, for the treatment of lymphoma or autoimmune disease is safe during pregnancy.– However, these same groups conducted follow-up studies that identified the drug within the nasal cavity of newborns born to pregnant macacos treated during pregnancy. Therefore, this treatment path may be harmful to immune development during the first months of life of the newborn. With the development of immune suppression therapies that demonstrate success for the treatment of cancer and lymphomas, if we can prove that they are safe for both the mother and the baby before and after, should we consider the use of such therapies for PE women? This research was supported by the National Institutes of Health HL105324, , HL51971, HL78147, and HD067541. The authors have no conflicts of interest to reveal. ReferencesFormats: Share , 8600 Rockville Pike, Bethesda MD, 20894 USA
Loading...Information about the Coronavirus Novel 2019. Information about coronavirus 2019 (COVID-19). HELLP Syndrome What is HELLP syndrome? HELLP syndrome is a rare but life-threatening condition during pregnancy. It causes red blood cells to break down. It also causes problems with the liver, bleeding, and blood pressure. It is often linked to preeclampsia and eclampsia. It often develops before delivery. But it can also happen after delivery. The letters in the word HELLP are held: Hemolisis. This is the collapse of red blood cells. elevated liver enzymes. Damage to liver cells causes changes in the way the liver works. Low platelets. Platelets are cells in the blood that help the blood coagulate to control the bleeding. What causes HELLP syndrome? Medical care providers don't know what causes HELLP syndrome. Who is at risk for HELLP syndrome? It is more likely that you get HELLP syndrome if: Having preeclampsia or eclampsia during pregnancy You had another pregnancy with HELLP syndrome Having a sister or mother who had HELLP syndrome What are the symptoms of HELLP syndrome? These are the most common symptoms of HELLP syndrome: Pain on the upper right side of your belly (abdomen) or around your stomach Nausea or vomitingHeadacheHigh blood pressure High blood pressure Protein in your urineSwelling (edema)The symptoms of HELLP syndrome may look like other health conditions. These include other high blood pressure problems in pregnancy. Always consult your healthcare provider for a diagnosis. How is HELLP syndrome diagnosed? Your health care provider will review your health history and perform a physical exam. Other tests include: Blood pressure measurement Red blood cell count Blood tests for enzymes showing cell damageMeasure of bilirubin level. This is a substance made by the collapse of red blood cells. Liver Function Tests Platelet count urine tests for protein How is HELLP syndrome treated? Treatment may include: Rest of bed, either at home or at the hospitalBlood transfusions for severe anemia and low platelet countMedicine to prevent seizures Medications to lower blood pressureHospital patient with fetal monitoring. This includes: Stress-free tests. This test measures the fetal heart rate when the baby moves. Biophysical profile. This test combines non-stress test with ultrasound to see the developing baby. Doppler flow studies. This is a type of ultrasound that uses sound waves to measure blood flow through a blood vessel. Liver, urine, and blood lab tests that may indicate whether HELLP syndrome is getting worseCorticosteroid medications to help the baby's lungs mature for childbirth You can deliver your baby early if HELLP syndrome gets worse and puts the health of you or your baby in danger. What are the possible complications of HELLP syndrome? Complications may include: Poor blood flow to your organs SeizuresAnemiaSludge clotting problems Placenta problems Liver problems Fluid construction in your lungs Preview delivery If HELLP syndrome is severe, you and your baby may be in danger. You may need to deliver the baby early to prevent further problems. It may take several days after delivery to recover from HELLP syndrome. What can I do to prevent HELLP syndrome? See signs of HELLP syndrome can help prevent some complications. Learning about warning signs can help you get early treatment and prevent the disease from getting worse. Key points about HELLP syndromeHELLP syndrome is a problem that threatens life during pregnancy. It can cause problems with the liver, bleeding, and blood pressure. Medical care providers don't know what causes HELLP syndrome. You may need to deliver your baby early. Find out if you are at risk for HELLP syndrome can help prevent some complications from the disease. Next steps Tips to help you get the most out of a visit to your healthcare provider: Know the reason for your visit and what you want to happen. Before your visit, write questions you want to answer. Bring someone with you to help you ask questions and remember what your provider tells you. In the visit, type the name of a new diagnosis, and any new medication, treatment or test. Also write any new instruction your provider gives you. Know why a new medication or treatment is prescribed, and how it will help you. He also knows what side effects are. Ask if your condition can be treated in other ways. Know why a test or procedure is recommended and what the results can mean. Know what to expect if you do not take the medication or have the test or procedure. If you have a follow-up appointment, type the date, time and purpose for that visit. You know how you can contact your provider if you have questions. Related Topics Related Links© 2021 Stanford Children's HealthAboutConnectFindAlsoInform us on:

HELLP Syndrome: Recognition and Perinatal Management - American Family Physician
Some of the differences seen in early and late HELLP syndrome and acute... | Download Table
PREECLAMPSIA – The continued nightmare – The Alexa Trust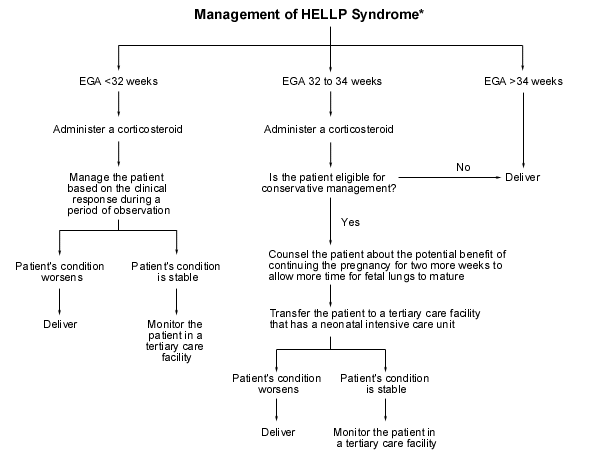
HELLP Syndrome - Physiopedia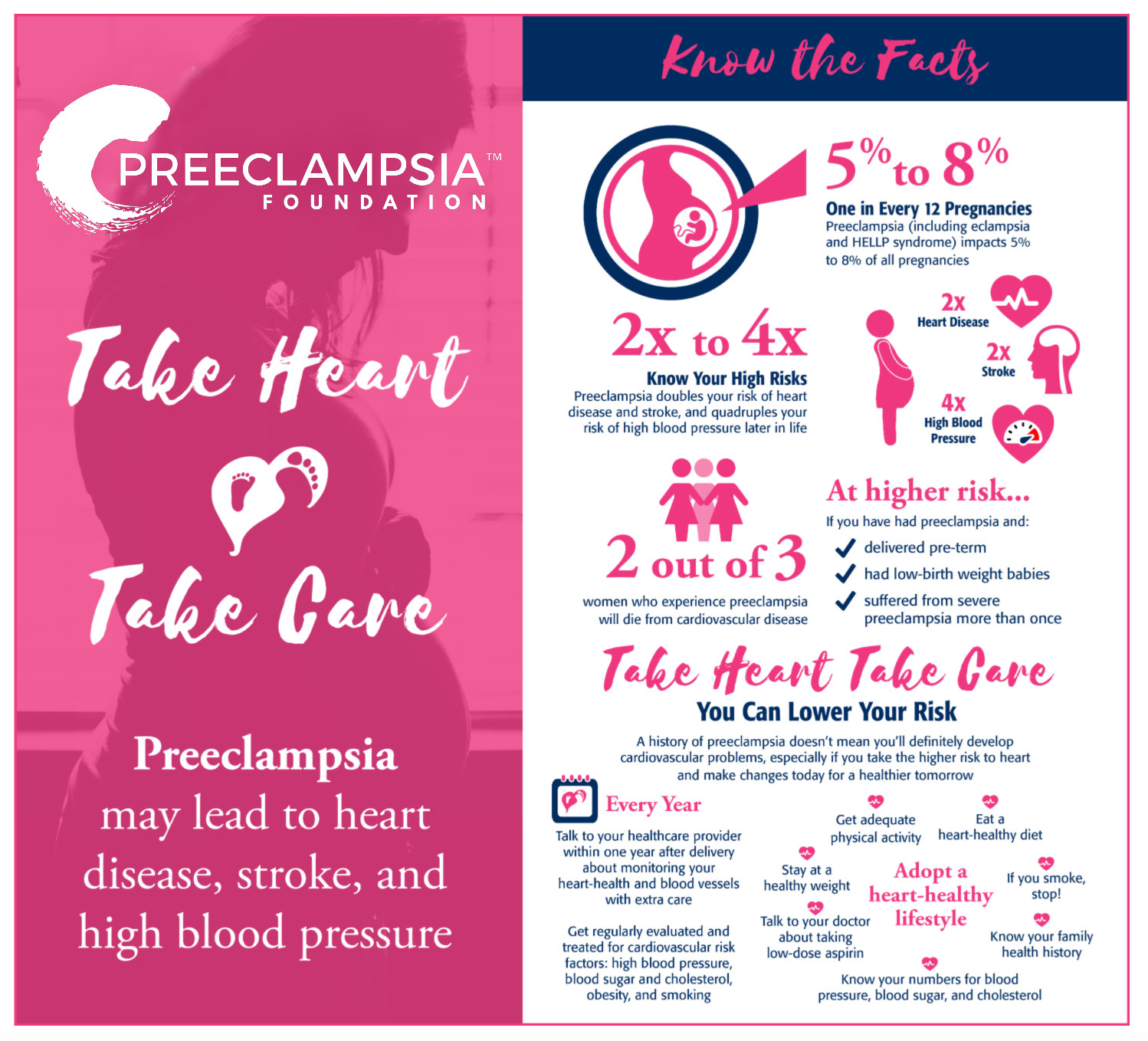
Heart Disease & Stroke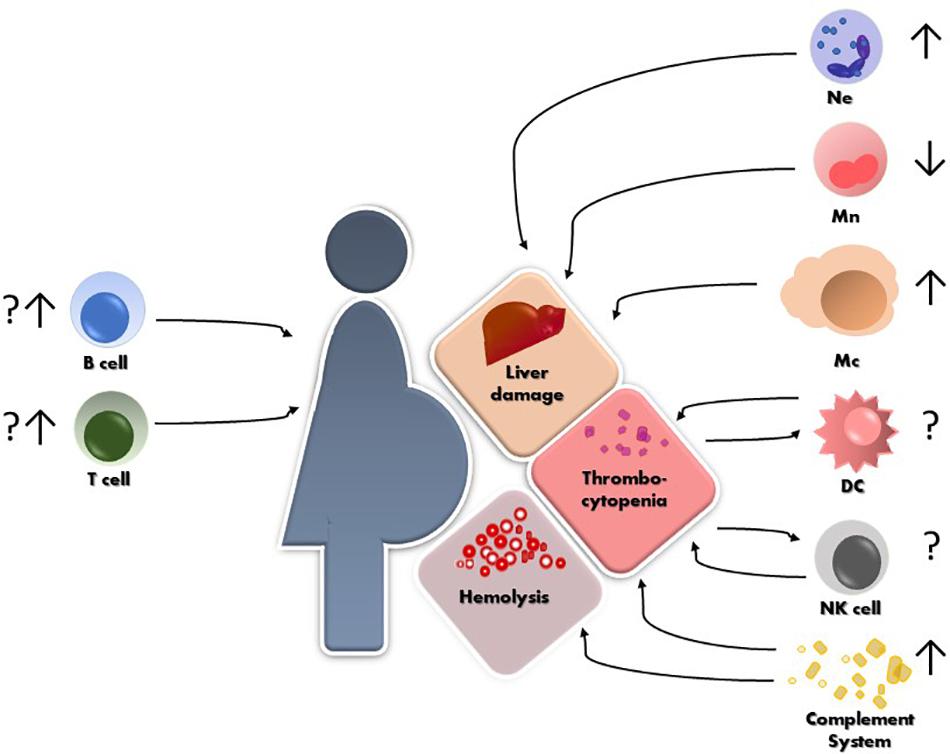
Frontiers | Innate and Adaptive Immune Responses in HELLP Syndrome | Immunology
Forest plot showing relative risk of HELLP syndrome and/or eclampsia... | Download Scientific Diagram
HELLP Syndrome: Risk Factors, Symptoms, and Treatment
Preeclampsia & HELLP - EMCrit Project
Liver disease in pregnancy - Cancer Therapy Advisor
HELLP Syndrome
The Effects of Maternal HELLP Syndrome on the Neonate
Two systems used to classify HELLP syndrome | Download Table
Understanding and managing HELLP syndrome: The ... - hapMD
Preeclampsia and Cerebrovascular Disease | Hypertension
Postpartum Preeclampsia: Moms are Still at Risk After Delivery
Baby V's Birth Story with HELLP Syndrome (Long) : BabyBumps
The HELLP syndrome: Clinical issues and management. A Review – topic of research paper in Clinical medicine. Download scholarly article PDF and read for free on CyberLeninka open science hub.![PDF] HELLP syndrome (hemolysis, elevated liver enzymes, and low platelets) pathophysiology and anesthetic considerations. | Semantic Scholar PDF] HELLP syndrome (hemolysis, elevated liver enzymes, and low platelets) pathophysiology and anesthetic considerations. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/5eec99373d1820adb3c7bf7a4dc9cdfc7a363429/3-TableI-1.png)
PDF] HELLP syndrome (hemolysis, elevated liver enzymes, and low platelets) pathophysiology and anesthetic considerations. | Semantic Scholar
Effect Sizes (Changes From Baseline) in the Markers of HELLP Syndrome... | Download Table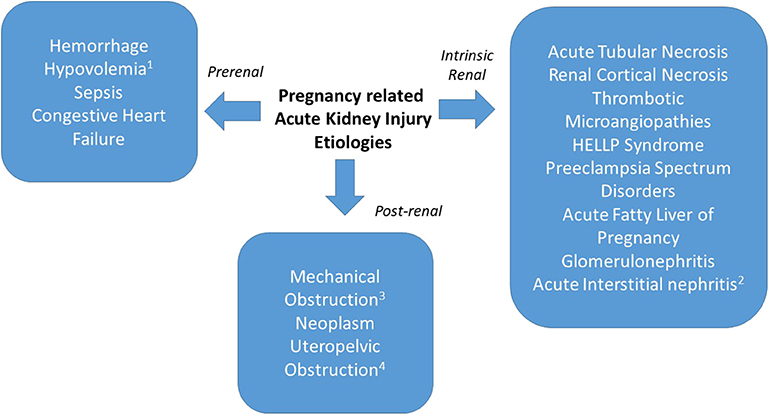
Frontiers | Acute Kidney Injury in Pregnancies Complicated With Preeclampsia or HELLP Syndrome | Medicine
A Model for the HELLP Syndrome: The Maternal Experience - Journal of Obstetric, Gynecologic & Neonatal Nursing
Preeclampsia & HELLP - EMCrit Project
What is HELLP syndrome? Symptoms, treatments and more
The hellp syndrome clinical issues and management. a review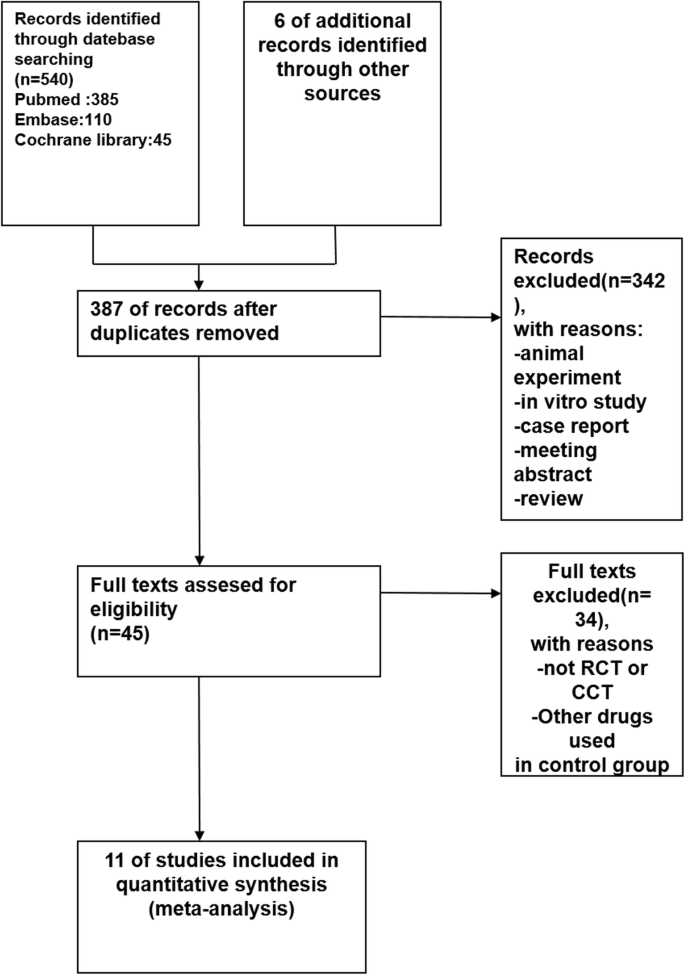
Effect of HELLP syndrome on acute kidney injury in pregnancy and pregnancy outcomes: a systematic review and meta-analysis | BMC Pregnancy and Childbirth | Full Text
PDF) HELLP SYNDROME – A diagnostic dilemma
Postpartum Preeclampsia: Moms are Still at Risk After Delivery
Prepregnancy and early pregnancy calcium supplementation among women at high risk of pre-eclampsia: a multicentre, double-blind, randomised, placebo-controlled trial - The Lancet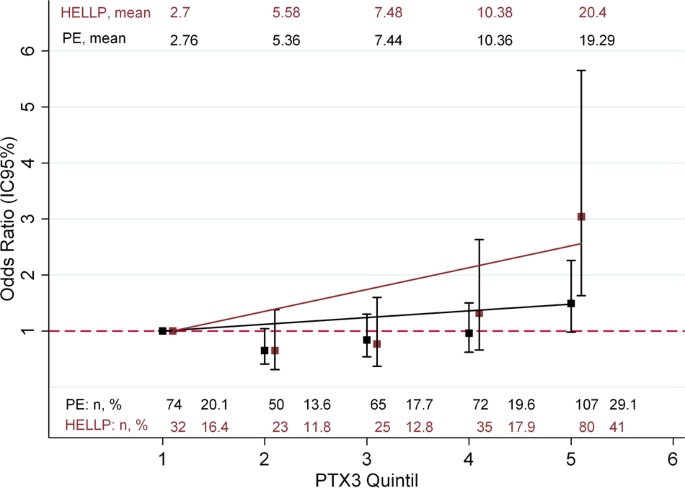
Pentraxin-3 is a candidate biomarker on the spectrum of severity from pre-eclampsia to HELLP syndrome: GenPE study | Hypertension Research
Partial HELLP Syndrome: maternal and perinatal outcome
HELLP Syndrome: Risk Factors, Symptoms, and Treatment
Pathogenesis of the syndrome of hemolysis, elevated liver enzymes, and low platelet count (HELLP): a review - European Journal of Obstetrics and Gynecology and Reproductive Biology
Hellp Syndrome disease: Malacards - Research Articles, Drugs, Genes, Clinical Trials
What Is HELLP Syndrome? Ob-Gyns Explain Pregnancy Complication | Health.com
Acute Kidney Injury in Pregnancy: The Changing Landscape for the 21st Century - ScienceDirect
HELLP Syndrome during Pregancy: Reasons, Symptoms & Treatment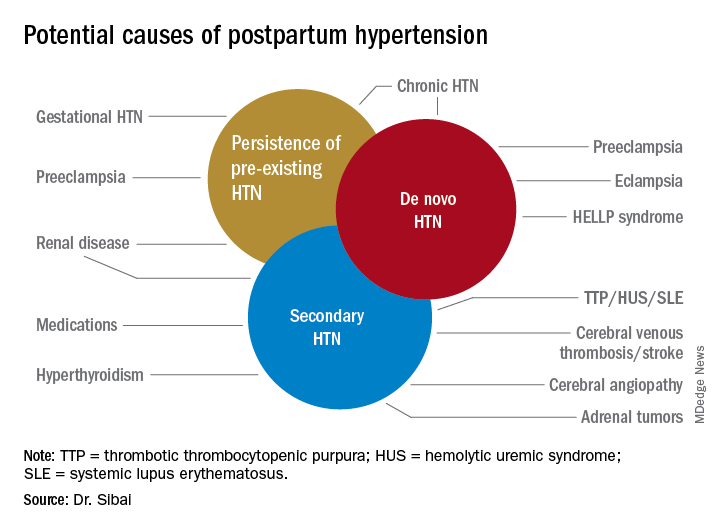
Recognition, evaluation, and management of postpartum hypertension | MDedge ObGyn
HELLP Syndrome and the Effects on the Neonate | Springer Publishing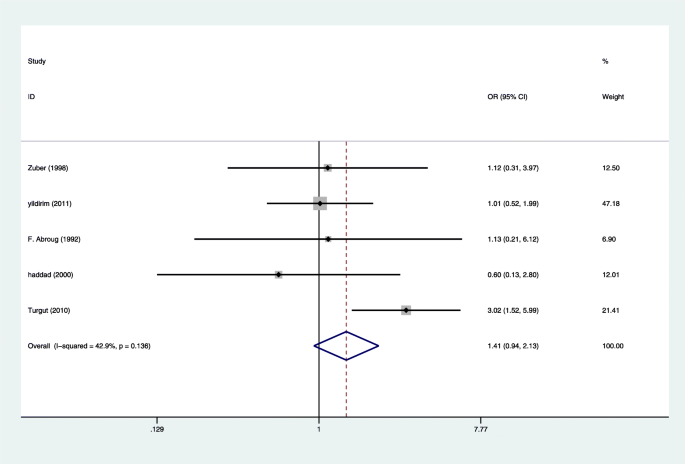
Effect of HELLP syndrome on acute kidney injury in pregnancy and pregnancy outcomes: a systematic review and meta-analysis | BMC Pregnancy and Childbirth | Full Text
 What Is HELLP Syndrome? How Can It Affect Mothers and Babies?
What Is HELLP Syndrome? How Can It Affect Mothers and Babies?















![PDF] HELLP syndrome (hemolysis, elevated liver enzymes, and low platelets) pathophysiology and anesthetic considerations. | Semantic Scholar PDF] HELLP syndrome (hemolysis, elevated liver enzymes, and low platelets) pathophysiology and anesthetic considerations. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/5eec99373d1820adb3c7bf7a4dc9cdfc7a363429/3-TableI-1.png)


















Posting Komentar untuk "hellp syndrome after effects"